Objectives
Upon completion of this module topic, you should:
- Be able to use electrophoresis techniques to analyze, purify and identify proteins by using the technique to separate proteins according to their electrophoretic mobility to estimate their molecular weights.
- Be able to prepare a sodium dodecyl sulfate (SDS) polyacrylamide gel (PAG) for protein electrophoresis (E). (SDS-PAGE)
- Be able to prepare protein samples from tissue and cell samples for electrophoresis (SDS-PAGE) using protein extraction, homogenization, denaturation and preparation techniques.
- Be able to assemble to SDS-PAGE apparatus for running a protein gel.
- Be able to load prepared protein samples on to a polyacrylamide gel for electrophoresis.
- Be able to run SDS-PAGE using electrical current generated through a power supply.
- Be able to conduct the Western Blot technique following SDS-PAGE to detect specific proteins in samples of tissue homogenates or extracts.
- After conducting SDS-PAGE, be able to transfer separated proteins to a nitrocellulose or PVDF membrane by appropriately assembling the Western Blot transfer apparatus and preparing (equilibrating and charging) the transfer membrane.
- Be able to probe the proteins transferred to the nitrocellulose or PVDF membrane using antibodies specific to the target protein (primary antibody specific to the target protein and a secondary antibody containing a detection signal such as horseradish peroxidase specific to the primary antibody bound to the target protein).
- Be able to analyze the probed transfer membrane using Ultraviolet fluorescence or gel staining techniques to display your probed protein target(s).
- Be able to interpret your Western Blot results by estimating the size(s) of your detected protein(s) from your samples.
- Be able to explain and perform an Enzyme-Linked Immunosorbant Assay (ELISA) to detect the presence of a particular antibody or an antigen in a sample.
- Understand the mechanisms of the ELISA technique and its important applications to medicine and disease diagnosis.
Part a
SDS-PAGE
This is Part A,SDS-PAGE, under the module topic Protein Techniques. This topic part has two sections to review: Content Tutorial & Animations.
Content Tutorial
Protein Identification Annenberg Media Rediscovering Biology & Modern Experimental Biochemistry, Boyer, R
a. Click on the following link to read about how the polyacrylamide gel electrophoresis technique is used to separate and identify proteins.
2-D Gel Electrophoresis to Identify Cellular Proteins: (www.learner.org, Multimedia Page)
Key Terms: polyacrylamide gel electrophoresis (PAGE), 2-D Gel Electrophoresis, isoelectric point
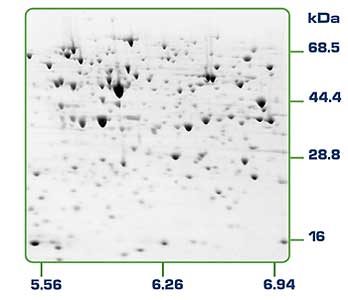
2-D Gel of Proteins: Annenberg Media Rediscovering Biology
Figure 4. Haemophilus influenzae cell proteins separated by 2D gel electrophoresis. The basic proteins are to the right of the gel and the acidic proteins to the left. High molecular weight proteins are to the top of the gel. (Annenberg Media, Rediscovering Biology)
b. Click on this additional link to learn about how mass spectrometry is used to identify proteins.
Mass Spectometry to Identify Cellular Proteins: (www.learner.org, Multimedia Page)
Key Terms: mass spectrometry, peptide mass mapping, protein fingerprinting
c. The following excerpt provides a description about how electrophoresis is used as a protein techniques tool for separating proteins and estimating their molecular masses.
Protein Identification & SDS-PAGE: Modern Experimental Biochemistry, Boyer, R. “In review, electrophoresis is an analytical tool by which biochemists can examine the movement of charged molecules in an electric field. Modern electrophoretic techniques use a polymerized gel-like matrix as a support medium. The sample to be analysed is applied to the medium as a spot or thin band. The migration of molecules is influenced by the applied electric field; the rigid, mazelike matrix of the gel support; and the size, shape, charge, and chemical composition of the molecules to be separated. Electrophoresis, is a relatively rapid and convenient technique that is capable of analyzing and purifying several different types of biomolecules, but especially proteins and nucleic acids. The agarose electrophoretic technique (described in module subset II.-a) for nucleic acids is not applicable to the measurement of the molecular weights of biological molecules such as proteins, because mobility through the gel is influenced by both charge and size. However, if protein samples are treated so that they have a uniform charge, then electrophoretic mobility depends primarily on size. The molecular weights of proteins may be estimated if they are subjected to electrophoresis in the presence of a detergent, sodium dodecyl sulfate (SDS), and a disulfide bond reducing agent, mercaptoethanol. This method is often called “denaturing electrophoresis”. When protein molecules are treated with SDS, the detergent disrupts the secondary, tertiary, and quarternary structure to produce linear polypeptide chains coated with negatively charged SDS molecules. The presence of mercaptoethanol assists in protein denaturation by reducing all disulfide bonds. The detergent binds to hydrophobic regions of the denatured protein chain in a constant ratio of about 1.4 grams of SDS per gram of protein. The bond detergent molecules carrying negative charges mask the native charge of the protein. Therefore, polypeptide chains of a constant charge/mass ratio and uniform shape are produced. The electrophoretic mobility of the SDS-protein complexes is influenced primarily by molecular size: the larger molecules are slowed down by the molecular sieving effect of the gel, and the smaller molecules have greater mobility. Empirical measurements have shown a linear rel ationship between the log molecular weight and the electrophoretic mobility. To provide an example, in practice, a protein of unknown molecular weight and subunit structure is treated with 1% SDS and 0.1 M mercaptoethanol in electrophoresis buffer. A standard mixture of proteins with known molecular weights must also be subjected to electrophoresis under the same conditions. Two sets of standards are commercially available, one for low-molecular-weight proteins (molecular weight range 14,000 to 100,000) and one for high-molecular weight proteins (45,000 to 200,000). SDS-PAGE is valuable for estimating the molecular weight of protein subunits. This modification of gel electrophoresis finds its greatest use in characterizing the sizes and different types of subunits in oligometric proteins. SDS-PAGE is limited to a molecular weight range of 10,000 to 200,000. Gels of less than 2.5% acrylamide must be used for determining molecular weights above 200,000, but these gels do not set well and are very fragile because of minimal cross-linking. A modification using gels of agarose-acrylamide mixtures allows the measurement of molecular weights above 200,000.” (Boyer, R., Modern Experimental Biochemistry, Third Edition. Addison Wesley Longman, Inc. 2000) *Need permission from the publisher
Additional Tutorials:
1. Protein Techniques: DNA and Proteins Basic Technology: SDS-PAGE
University of Calgary Biotechnology Training Centre
Protein Techniques PDF
2. SDS-PAGE:
Rice University – Experimental Biosciences Click on the following link to view a tutorial about the steps involved in SDS-PAGE. Use the purple arrows on the right side of the webpage to navigate through the tutorial. The tutorial is structured into the following four sections:
- Introduction to SDS-PAGE
- Preparing SDS-PAGE gels
- Preparing protein samples for electrophoresis
- Assembling, loading and running gels
Introduction to SDS-PAGE (www.ruf.rice.edu, Multimedia Page)
Animations
1. Annenberg Media, Rediscovering Biology to see depictions relating to how mass spectrometry is used as a tool for protein identification. Mass Spectrometer A depiction of what happens inside a mass spectrometer.
- View Quicktime Movie (www.learner.org, Multimedia Page)
2. SDS-PAGE Animation Biosolutions “SDS-PAGE, sodium dodecyl sulfate polyacrylamide gel electrophoresis, is a technique used in biochemistry, genetics and molecular biology to separate proteins according to their electrophoretic mobility (a function of length of polypeptide chain or molecular weight as well as higher order protein folding, posttranslational modifications and other factors).” Biosolutions. Text: View the following document to read a description about the SDS-PAGE Procedure from Biosolutions. Procedure description:SDS-PAGE
- SDS-PAGE Animation (www.biosolutions.info, Multimedia Page)
Part b
Western Blot
This is Part b, Western Blot, under the module topic Protein Techniques. This topic part has three sections to review: Content Tutorial, Animations, Activities.
Content Tutorial
Protein Techniques Continued: DNA and Proteins Basic Technology: Western Blotting
Click on the following link to view the University of Calgary tutorial with graphic demonstrations about conducting the Western Blot technique.
- Western Blot Technique (heart.jabsom.hawaii.edu, PDF Document)
Animations
1. Biosolutions: Western Blot Animation
“The western blot (alternately, immunoblot) is a method of detecting specific proteins in a given sample of tissue homogenate or extract. It uses gel electrophoresis to separate native or denatured proteins by the length of the polypeptide (denaturing conditions) or by the 3-D structure of the protein (native/ non-denaturing conditions). The proteins are then transferred to a membrane (typically nitrocellulose or PVDF), where they are probed (detected) using antibodies specific to the target protein. There are now many reagent companies that specialize in providing antibodies (both monoclonal and polyclonal antibodies) against many thousands of different proteins. Commercial antibodies can be expensive, though the unbound antibody can be reused between experiments. This method is used in the fields of molecular biology, biochemistry, immunogenetics and other molecular biology disciplines. Other related techniques include using antibodies to detect proteins in tissues and cells by immunostaining and enzyme-linked immunosorbent assay (ELISA). The method originated from the laboratory of George Stark at Stanford. The name western blot was given to the technique by W. Neal Burnette and is a play on the name Southern blot, a technique for DNA detection developed earlier by Edwin Southern. Detection of RNA is termed northern blotting.” (Biosolutions – video) Text: View the following document to read a description about the steps involved in a Western Blot from Biosolutions.
Procedure description: Western Blot
- Western Blot Animation (www.biosolutions.info, Multimedia Page)
2. WH Freeman Animation: Western Blotting (Immunoblotting)
You may view the animated tutorials in a slide-by-slide version as you navigate through or in a narrated version that navigates for you.
- Animation (bcs.whfreeman.com, Multimedia Page)
Activities
Click on the following link to complete the “Introduction to Western Blot Activity”. Navigate the webpage by clicking on the links under the “Contents” section. The links include the following, Background, Visualization of the Western Blot, Viral Proteins and Band Pattern Interpretation and Test Yourself (4 Problems).
- Western Blotting Continued (www.biology.arizona.edu, Multimedia Page)
Part c
Antibodies & Antigens
This is Part c, Protein Expression, under the module topic Protein Techniques. This topic part has three sections to review: Content Tutorial, Animations & Activities.
Content Tutorial
Task: Visit the following webpage designed by the Arizona Biology Project to learn about the structure of an antibody and how that structure determines how the antibody will interact with an antigen. The tutorial provides information in the following format: Introduction, Antibody/Antigen Interaction, and Antibody/Antigen Interaction – A Closer Look.
- Tutorial (www.biology.arizona.edu, Multimedia Page)
Animations
1. The Arizona Biology Project: Antibody Structure Tutorial – Animations The two animation links below reinforce the content presented in the Antibody Structure Tutorial you just completed.
a. This first animation displays the overlapping beta sheets of the FR (framework) region joined to the HV (hypervariable) region of the antibody.
- Animation: FR and HV regions of antibody (www.biology.arizona.edu, Multimedia Page)
b. This second animation displays the three dimensional interaction between an antibody and an antigen. It is the HV regions of the antibody that interact with the surface of the antigen. Animation: Antigen interacts with HV region c. This third animation displays a molecular simulation of a specific example of an antibody interacting with an antigen. The anitigen is hen egg white lysozyme and it contains the unique structure of the amino acid, glutamine at position 121 that the antibody’s HV region interacts with.
- Animation: Gln 121 of lysozyme surrounded by amino acid residues of antibody(www.biology.arizona.edu, Multimedia Page)
2. Antibody Animated Tutorial – Making a Monoclonal Antibody (Sumanas Inc.) You may view the animated tutorials provided on the website in a slide-by-slide version that you navigate through or in a narrated version that navigates for you.
- Animation (www.sumanasinc.com, Multimedia Page)
3. Monoclonal Antibody Production Animations from Raven, Johnson, Losos, & Singer, Biology, 7th Edition, 2005 Click on the following link to view an animation about how monoclonal antibodies are produced. The animation provides a narration as well as guided text to support the simulation.
- Animation (highered.mcgraw-hill.com, Multimedia Page)
4. IgE Mediated (Type I) Hypersensitivity Animations from Raven, Johnson, Losos, & Singer, Biology, 7th Edition, 2005 Click on the following link to view an animation about how hypersensitivities (or allergic responses) are mediated by one specific type of antibody known as Immunoglobulin (Ig) E. The animation depicts the interaction between antibodies (specifically IgE) and antigens (specifically allergens) and also depicts the roles of and interactions between B and T cells of the immune system in the hypersensitivity reaction. The animation provides a narration as well as guided text to support the simulation.
- Animation (highered.mcgraw-hill.com, Multimedia Page)
Activities
The Arizona Biology Project: Antibody Tutorial – Problem Set Instructions: Click on the following link to complete the Arizona Biology Project, Antibody Structure Problem Set. There are 8 multiple-choice problems to complete. The correct answers are reinforced with a brief explanation and the incorrect answers are linked to tutorials to help solve the problem. It is recommended that you complete the tasks for the Antibody Structure Tutorial from the Content Tutorial and Animations sections above prior to completing the problem set.(Reference: Arizona Biology Project)
- Problem Set (www.biology.arizona.edu, Multimedia Page)
Part d
ELISA
This is Part d, ELISA, under the module topic Protein Techniques. This topic part has three sections to review: Introduction, Animations & Activities.
Content Tutorial
“ELISA, Enzyme ImmunoAssay or EIA, is a biochemical technique used mainly in immunology to detect the presence of an antibody or an antigen in a sample. The ELISA has been used as a diagnostic tool in medicine and plant pathology, as well as a quality control check in various industries. In simple terms, in ELISA an unknown amount of antigen is affixed to a surface, and then a specific antibody is washed over the surface so that it can bind to the antigen. This antibody is linked to an enzyme, and in the final step a substance is added that the enzyme can convert to some detectable signal. Thus in the case of fluorescence ELISA, when light of the appropriate wavelength is shone upon the sample, any antigen/antibody complexes will fluoresce so that the amount of antigen in the sample can be inferred through the magnitude of the fluorescence. Performing an ELISA involves at least one antibody with specificity for a particular antigen. The sample with an unknown amount of antigen is immobilized on a solid support (usually a polystyrene microtiter plate) either non-specifically (via adsorption to the surface) or specifically (via capture by another antibody specific to the same antigen, in a “sandwich” ELISA). After the antigen is immobilized the detection antibody is added, forming a complex with the antigen. The detection antibody can be covalently linked to an enzyme, or can itself be detected by a secondary antibody, which is linked to an enzyme through bioconjugation. Between each step the plate is typically washed with a mild detergent solution to remove any proteins or antibodies that are not specifically bound. After the final wash step the plate is developed by adding an enzymatic substrate to produce a visible signal, which indicates the quantity of antigen in the sample. Older ELISAs utilize chromogenic substrates, though newer assays employ fluorogenic substrates enabling much higher sensitivity. The ELISA test, or the enzyme immunoassay (EIA), was the first screening test commonly employed for HIV. It has a high sensitivity. In an ELISA test, a person’s serum is diluted 400-fold and applied to a plate to which HIV antigens have been attached. If antibodies to HIV are present in the serum, they may bind to these HIV antigens. The plate is then washed to remove all other components of the serum. A specially prepared “secondary antibody” — an antibody that binds to other antibodies — is then applied to the plate, followed by another wash. This secondary antibody is chemically linked in advance to an enzyme. Thus the plate will contain enzyme in proportion to the amount of secondary antibody bound to the plate. A substrate for the enzyme is applied, and catalysis by the enzyme leads to a change in color or fluorescence. ELISA results are reported as a number; the most controversial aspect of this test is determining the “cut-off” point between a positive and negative result.” (Reference: Biosolutions, http://bioisolutions.blogspot.com/2009/01/elisa.html)
Animation
1. ELISA Animation: Reference – Biosolutions
View the following document to read a description about the steps involved in a Western Blot from Biosolutions.
- Various Types of ELISA Assays (heart.jabsom.hawaii.edu, HTML Page)
Click on the following link to view an animation of the ELISA Assay and a video demonstration of a scientist performing an ELISA Assay in the laboratory.
- Types of ELISA Assays (www.bioisolutions.info, Multimedia Page)
2. ELISA Animated Tutorial: Reference – Sumanas Inc. You may view the animated tutorials in a slide-by-slide version that you navigate through or in a narrated version that navigates for you.
- ELISA Tutorial (www.sumanasinc.com, Multimedia Page)
3. ELISA Animation: Reference – Wiley Science The following website provides a step by step animation of the ELISA Assay. You can navigate through the technique steps by clicking on the yellow arrow on the bottom right-hand area of the website.
- Elisa Animation (www.wiley.com, Multimedia Page)
Activities
1. Arizona Biology Project – ELISA Assay Click on the link provided below to learn about the ELISA Assay. You will complete an interactive content tutorial activity and then complete the problem set provided (4 problems). “Western Blot is used as a confirmation of the ELISA Assay for HIV. To learn more about this, visit The Biology Project module on ELISA.” (Reference, The Arizona Biology Project)
- Activity: The Elisa Assay (www.biology.arizona.edu, Multimedia Page)
2. Virtual Laboratory Experiment Howard Hughes Medical Institute – The Virtual Immunology Lab
Concepts Covered: Howard Hughes Medical Institute – The basis of humoral immunity – The foundation for ELISA – Potential errors in conducting an ELISA – Sensitivity and specificity of a diagnostic test
Virtual Laboratory Experiment Overview In this virtual laboratory experiment you will take on the role as a manager of a clinical laboratory. You will be testing blood samples from three different patients that are being screened for the disease systemic lupus erythematosus (SLE). In order to test the samples you will conduct an ELISA Assay of the samples to screen for the presence of antibodies that coincide with the disease. You will begin by reading through the “Diagnosis” section, which explains information relating to the ELISA Assay technique and provides information about potential problems that may arise throughout the experiment as well as limitations that accompany the test. Next, you will explore important information about the experiment that you will conduct in the “Background” section. You will use the laboratory notebook to view the objective for the experiment as well as 11 subsequent protocol steps. You can respond to the questions proposed through each step of the protocol to ensure your understanding of the mechanisms and tasks involved in each step. To the left of the screen you can view the virtual laboratory room and equipment that you will be operating with and manipulating throughout the virtual experiment. It is important to know that as you continue through the virtual laboratory experiment your laboratory notebook steps on the right side of the webpage will follow in order automatically. There is also a glossary section that provides useful information about the laboratory and medical terminology your will encounter throughout the experiment. *Reminder, do not skip steps throughout the experiment, it can alter your results and result in human error. You can view a summary report of your virtual laboratory work when you have completed the experiment.
- Virtual Immunology Lab (www.hhmi.org, Multimedia Page)
Part e
Protein Microarrays
This is Part E, Protein Microarrays, under the module topic Protein Techniques. This topic part has one sections to review: Content Tutorial.
Content Tutorial
Large-scale Protein Analysis
1. Annenberg Media, Rediscovering Biology: Protein Microarrays
Click on the following link to learn about the protein microarray technique and how it applies to the large-scale study of proteins, similar to DNA microarrays used for the large-scale study of nucleic acids. View only the one electronic text page that appears when you access the site.
- Protein Microarray Technique (www.learner.org, Multimedia Page)
2. Harvard University Department of Chemistry and Biological Chemistry, Macbeath Lab: Description of Protein Microarray Technology
Click on the following link to learn about Protein Microarray Technology. The tutorial is accompanied by graphics illustrating various aspects relating to the technique. View only the one electronic text page that appears when you access the site.
- Protein Microarray Technology (sysbio.harvard.edu, Multimedia Page)
*Note: For more content, animations and activities relating to Microarray Techniques and Technology see section II-c. of the Nucleic Acids Module.